Service
Study design
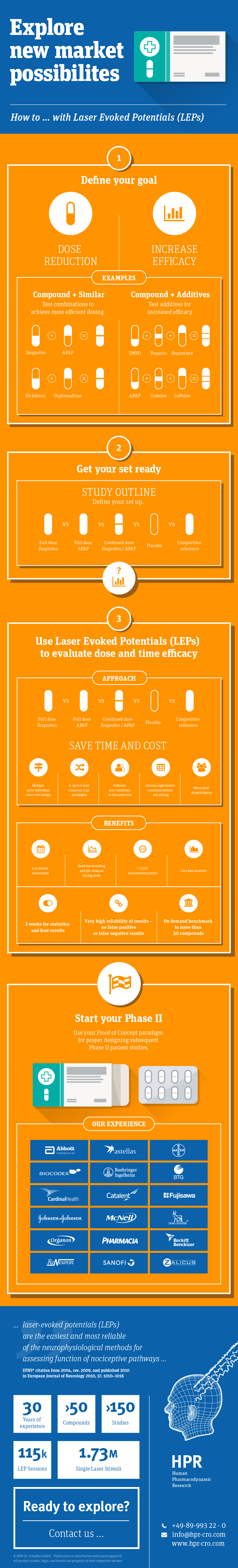
• Multiple intra-individual cross-over study designs
• 2- up to 6-way crossover trial paradigms
• also with different skin conditions in the same trial conduct
• 2- up to 6-way crossover trial paradigms
• also with different skin conditions in the same trial conduct
…this allows us to operate at well balanced time and cost aspects
Full Service
• Full-service-testing of diverse nociceptive conditions – within one study setting
• Minor pool of participants (e.g. about 16 to 30 subjects only)
• Study conduct in well-defined time-lines
Conditions
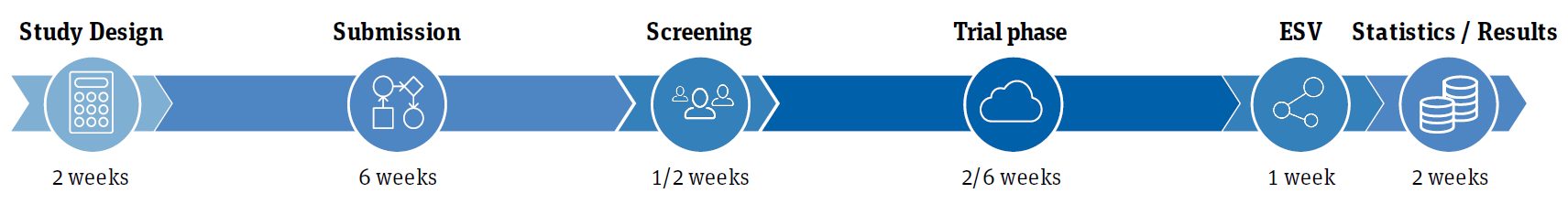
• 6-way (single-dose) trial in 6 weeks + Screening and ESV period
• 2 weeks for statistics and first results (with up to >2300 LEP sessions per study)
• Realtime recording and pre-analysis during study
• 2 weeks for statistics and first results (with up to >2300 LEP sessions per study)
• Realtime recording and pre-analysis during study
The setting
• Laser Evoked Potentials (LEP) from normal and sensitized skin conditions
(e.g. UVB- and capsaicin-irritation)
• Healthy volunteers (+ psychophysics/ electronic VAS/ QST & skin colorimetry)
• Validity & Comparability high – due to sophisticated (objective-quantitative) test paradigms and due to the high expertise with reference to results of former studies in many other marketed and experimental analgesic compounds
-resulting in a large data base