Algesimetry: quantitative, objective and subjective, nociceptive processing
Usefulness of the methods
In contrast to formerly used pain models, nociceptive relief measured with our methods correlates well with clinical results. Nevertheless our experimental approach in healthy volunteers does not serve as a substitute for clinical trials in patients. Our methods are, however, more accurate, faster and less expensive in trial conduct and in establishing dose-response and time-efficacy relationships of new drugs or of new drug formulations or drug combinations. This helps to save time and money in designing adequate future clinical/patient studies. Moreover our models can provide data for Proof of Concepts (PoC) promptly and for the discrimination of candidate drugs or drug combinations.
Principle of the methods
Standardised pain induction: Defined contact-free CO2 laser pulses of identical intensity are applied to normal or irritated skin (e.g. with preceding UV or capsaicin exposure) without any inherent placebo effect by the method itself (see Laser Principle).
Objective quantitative algesimetry: Nociceptive processing is assessed by averaged (radiant-heat) stimuli-related potentials from Vertex-EEG position (resulting LEP with the two main components N2/P2, see figure left).
Subjective quantitative algesimetry: In addition pain impression is evaluated by subjective rating (VAS, 100mm) and stimulus dependent psychophysics (e.g. quantitative sensory testing/ QST with diverse threshold determinations, e.g. using Weighted Needle Threshold/ WNT), as a bridge to patient’s subjective experience of clinical pain experience.
Induction of hyperalgesia: Exposure/ irritation of skin with acute or steady-state UV-B (predominantly peripheral hyperalgesia) or topical capsaicin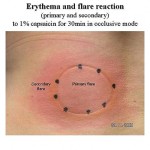 (mixed peripheral/ spinal/ central hyperalgesia with ongoing nociceptive input on spinal/ central level and induction of allodynia, for flare areas see figure left) facilitates the discrimination of moderately different analgesic effects with higher resolution (greater pharmacodiscriminativity).
(mixed peripheral/ spinal/ central hyperalgesia with ongoing nociceptive input on spinal/ central level and induction of allodynia, for flare areas see figure left) facilitates the discrimination of moderately different analgesic effects with higher resolution (greater pharmacodiscriminativity).
Concomitant measurement of anti-inflammatory effects: E.g. in case of NSAID compounds the erythema-reducing effects can be measured by quantitative skin reflection spectrometry/ SRS (on UV or capsaicin skin), using the a-value of CIE-Lab system (“redness”).
Reference data
Both central and peripheral analgesic/ anti-hyperalgesic as well as local analgesic drug effects were demonstrated and differentiated. More than 50 complex algesimetric studies were conducted up to now.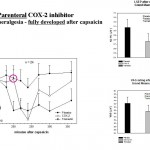 Data are available for systemically administered analgesics of different type and for antiepileptics, antidepressants, antihistamines, GABAergic compounds, NSAIDs (for effects of a selective parenteral COX-2 inhibitor see figure left) and opiates (for an example see figure below), as well as for topical analgesics, antihistamines and local anaesthetics (see our Database analgesics LSEP_HPR).
Data are available for systemically administered analgesics of different type and for antiepileptics, antidepressants, antihistamines, GABAergic compounds, NSAIDs (for effects of a selective parenteral COX-2 inhibitor see figure left) and opiates (for an example see figure below), as well as for topical analgesics, antihistamines and local anaesthetics (see our Database analgesics LSEP_HPR).
Advantages of the methods
These experimental approaches show – in contrast to pain models in patients – only very low variations. Thus already minor to moderate differences in the analgesic/ anti-hyperalgesic effect or in doses of analgesic compounds can be proven with small study groups at low cost. This is due to the following features of our methods:
- The nociceptive (radiant-heat) laser stimulus intensity will be individually kept constant over the complete study period for each trial participant.
- Stimulus intensity is adapted and fixed to individual pain threshold at screening.
- Stimuli are presented in random order and with dislocation after each shot to avoid local overstimulation, expectancy and habituation.
- Vigilance/attention is kept stable during LEP sessions by a permanent load with a pursuit tracking task and a superimposed aural (“white”) noise impact – also improving the subject’s distraction from the actual “pain events”.
- The objective, quantitative recordings of evoked potentials show by nature a distinctly smaller variation than subjective ratings of nociception/ pain.
- Trials can be run in an ethically acceptable intra-individual crossover design of smaller sample sizes than done in clinical (patient) studies, which are mostly done in parallel designs with a higher risk for biases.
- There is no inherent placebo effect with the laser set-up itself.
Up to 30 measurements per hour can be made (2 to 3 min sequence in EP-testing, equivalent to a High Throughput System/HTS). Thus the time course of an analgesic effect can be measured with high resolution (e. g. fast onset with immediate release formulations/ IR). Laser induced nociceptive impression, in contrast to that initiated by electrical stimulation or thermal heat-probes, is not subject to habituation and to influences of local hyperaemic respectively blood convection changes in the erythema areas. Therefore, long-lasting analgesic effects can be reliably measured too (e.g. of prolonged release preparations/ PR). Our short laser pulses (50-80 ms) selectively stimulate the thermo-nociceptors (TRPV 1-3) via heat-activated ion channels (A-delta and C-fibres). Thus, in contrast to electrical stimulation (with unspecific receptor and neuronal excitation and other biases), it is possible to measure predominantly peripheral nociceptive effects (e.g. in normal and in UV exposed skin) in addition and separately from more spinal/ central ones (e.g. in capsaicin-irritated skin), especially without inducing habituation by the type of stimulus per se.
Options
- Discrimination of moderate and moderately different anti-nociceptive/ anti- hyperalgesic effects of drugs, or doses of a drug
- Measurement of the time course of analgesic effects of drugs or special formulations (e.g. in fast onset or slow release paradigms)
- Establishing the dose-effect relationship of investigational compounds
- Assessment of the contribution of single components to the overall analgesic effect of drug combinations
- Concomitant assessment of effects on inflammation or vigilance/ performance or of pharmacokinetics (pd/pk)
The UVB and capsaicin model for induction of inflammatory and neurogenic hyperalgesia – combined with an objective-quantitative, anti-nociceptive measurement by laser-algesimetry
Usefulness of the methods
In many different painful and inflammatory clinical states neurogenic inflammation and acute inflammatory hyperalgesia play a significant role.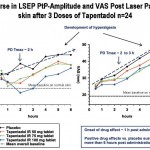 Acute pain is better mimicked by UV application with its primary peripheral, hyperalgesic components (for development and treatment in UV skin see figure left). Moreover all clinical diseases causing long-lasting pain involve central mechanisms of hyperalgesia. Application of capsaicin on or into the skin is a broadly accepted model for induction of neurogenic inflammation, hyperalgesia and allodynia, which involves a strong spinal/ central component. Any inhibitory effect of an investigational compound on neurogenic inflammation and acute inflammatory hyper-algesia would cast light on its potential efficacy in such different clinical settings.
Acute pain is better mimicked by UV application with its primary peripheral, hyperalgesic components (for development and treatment in UV skin see figure left). Moreover all clinical diseases causing long-lasting pain involve central mechanisms of hyperalgesia. Application of capsaicin on or into the skin is a broadly accepted model for induction of neurogenic inflammation, hyperalgesia and allodynia, which involves a strong spinal/ central component. Any inhibitory effect of an investigational compound on neurogenic inflammation and acute inflammatory hyper-algesia would cast light on its potential efficacy in such different clinical settings.
Principle of the methods
At HPR for skin sensitization local UVB-irradiation (about 290-320nm in visible spectral range) with 2- or 3-fold MED (individual Minimal Erythema Dose) is used for hyperalgesia induction on an area of about 5cm square, as well as topical capsaicin (in an occlusive dressing mode with 1% alcoholic capsaicin extract for 30min).
At HPR effects of an investigational drug on nociception and hyperalgesia due to neurogenic and inflammatory hyperalgesia can be measured using the objective  quantitative method of laser algesimetry (see separate description); further the redness of the UV- and capsaicin-induced inflammation can be quantified by skin reflection spectrometry (erythema/ redness) using the a-value of CIE-Lab system and the size of the capsaicin-induced flare area can be determined by computer planimetry.
quantitative method of laser algesimetry (see separate description); further the redness of the UV- and capsaicin-induced inflammation can be quantified by skin reflection spectrometry (erythema/ redness) using the a-value of CIE-Lab system and the size of the capsaicin-induced flare area can be determined by computer planimetry.
Reference data
At HPR the UV- and capsaicin model has been successfully used as a standard for the investigation of unspecific COX-inhibitors, selective COX2-, EP4- and TRPV1, VGCC inhibitors, centrally acting analgesics (opiates, opioids, cannabinoids), antidepressants, antiepileptics and H1-blocking agents (anti-histamines) etc..
Advantages of the methods
The state of UV-induced hyperalgesia is close to clinical conditions as acute post-traumatic and post-operative states (being a “real disease”, e.g. as sunburn), whereas capsaicin-induced hyperalgesia is closer to neuropathy and to clinical situations in which longer-lasting pain provokes a strong central component of hyperalgesia (by ongoing spinal input), which adds to the peripheral components of hyperalgesia.
Combining the UV or the capsaicin model with the laser algesimetry (with its additional kindling properties) or with mechano-evoked potentials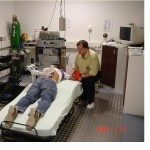 by an impactor (MEP, see set-up figure left) or QST (e.g. Weighted Needle), allows to imitate natural clinical states of acute/ inflammatory and longer-lasting pain and to simulate close to neuropathy, and to utilise the advantages of the laser algesimetry, i.e. its objectivity, reproducibility and repeatability in nociceptive stimulation and in the measurement of its central processing.
by an impactor (MEP, see set-up figure left) or QST (e.g. Weighted Needle), allows to imitate natural clinical states of acute/ inflammatory and longer-lasting pain and to simulate close to neuropathy, and to utilise the advantages of the laser algesimetry, i.e. its objectivity, reproducibility and repeatability in nociceptive stimulation and in the measurement of its central processing.
Options
Utilise the state of UV- and capsaicin-induced experimental hyperalgesia for the following measurements:
- Discrimination of moderate and moderately different nociceptive and anti- hyperalgesic effects of drugs or doses of drugs, especially with regard to major influences on peripheral and/or spinal-central hyperalgesia
- Measurement of the time course of anti-nociceptive/ anti-hyperalgesic effects of drugs or special formulations (immediate or prolonged release)
- Establishing the dose-effect relationship of investigational drugs
- Assessment of the contribution of single components to the overall analgesic effect of drug combinations
- Concomitant assessment of effects on inflammation, QST or vigilance and performance or of pharmacokinetics (pd/pk)